- VVT Medical
- >>
- Pages
- >>
- V-Block
ScleroSafe™ with V-Block™
ScleroSafe with V-Block is especially designed to simply, safely and effectively treat severely dilated and challenging Great Saphenous Vein (GSV) conditions, up to 14mm ID.
While all ScleroSafe products share the Double Lumen Catheter and Inverse Action Dual Syringe design concepts, V-Block products feature a unique embolization device which further enhances the safety and efficacy of treating larger vessels.
V-Block has CE mark, Amar (Israel), TGA (Australia) and UAE classification.
Indications
V-Block is designed to treat challenging GSV conditions, including GSV valve incompetence and Reflux. Indicated for: the occlusion of lower extremity veins with a diameter in the range of 4 to 14mm. Specifically, it is indicated for the occlusion of the Greater Saphenous Vein (GSV) as an adjunct tool for the treatment of venous insufficiency and saphenous reflux disease.
Configurations
ScleroSafe with V-Block can be used with the 10cc/20cc Dual Syringe, a proprietary, specially designed 6F (450mm) or 6F (650mm) catheter and a dedicated guide wire.
Dimensions
6F/ 450mm 6F/ 650mm
ScleroSafe™ with
V-Block™
ScleroSafe with V-Block is especially designed to simply, safely and effectively treat severely dilated and challenging Great Saphenous Vein (GSV) conditions, up to 14mm ID.
While all ScleroSafe products share the Double Lumen Catheter and Inverse Action Dual Syringe design concepts, V-Block products feature a unique embolization device which further enhances the safety and efficacy of treating larger vessels.
Indications
V-Block is designed to treat challenging GSV conditions, including GSV valve incompetence and Reflux. Indicated for: the occlusion of lower extremity veins with a diameter in the range of 4 to 14mm. Specifically, it is indicated for the occlusion of the Greater Saphenous Vein (GSV) as an adjunct tool for the treatment of venous insufficiency and saphenous reflux disease.
Configurations
ScleroSafe with V-Block can be used with the 10cc/20cc Dual Syringe, a proprietary, specially designed 6F (450mm) or 6F (650mm) catheter and a dedicated guide wire.
Dimensions
6F/ 450mm 6F/ 650mm
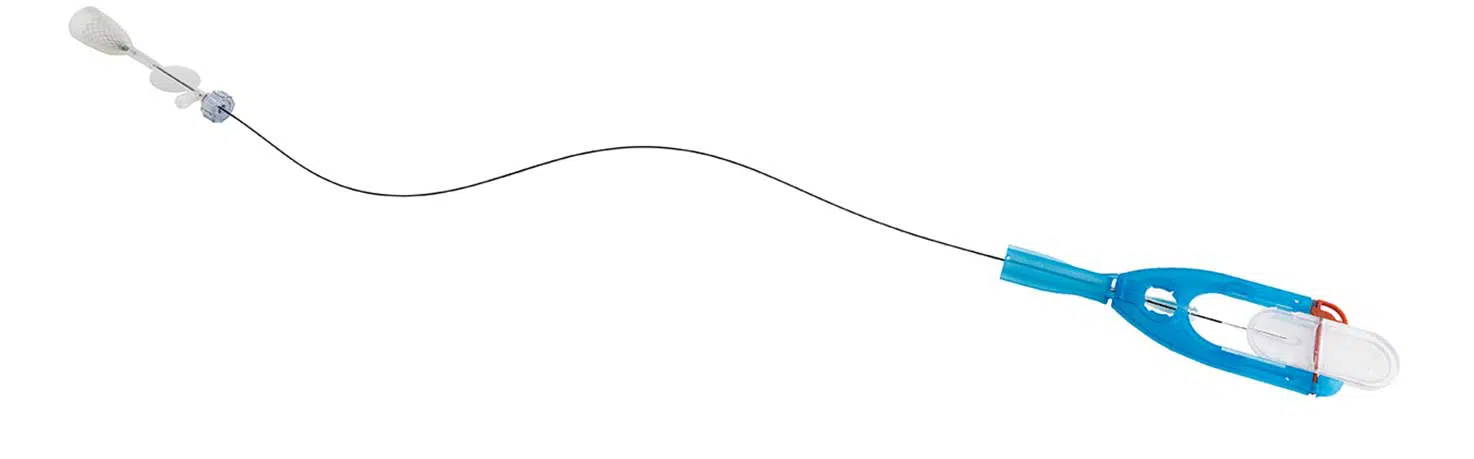

Embolization Safety Device
The embolization safety device is designed to block the blood flow within GSVs of larger diameters during ECA/Sclerotherapy procedures. The purpose of the device is to minimize the risk of upflow of the injected substance to the femoral vein and to the upper body. It is a conical, biomedical-grade, flexible nitinol frame covered with a polymer-coated mesh and is tested and approved as a permanent venous implant.
The V-Block device is integrated into the double lumen catheter. It is inserted into the GSV through a micro puncture access point, advanced upward, deployed and then secured 2 cm below the saphenofemoral junction. Its guidance and proper position are verified with ultrasound. Once deployed, VVT’s proprietary Dual Syringe discharges the sclerosant agent into the GSV while aspirating the residual blood, ultimately ablating and closing the faulty vessel. The entire procedure can be completed in less than 7 minutes.
Patient Testimonial
Eitan Machover
60 years old
Eitan underwent treatmant for varicose veins in both his legs. An hour later he already had lunch with friends, A year later he is feeling better than ever!
Meet our Groundbreaking Solutions
Please fill in the following details and one of our skilled professionals will get back to you
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.


